Tremendous advancements in science, public health, and material standards of living in recent decades means that people are living longer than ever before. In 1950, when the world’s population was 2.5 billion, life expectancy at birth was 46.5 years. In 2022, those figures rose to 8 billion and 71.2 years, respectively. By 2050, global life expectancy is projected to rise to 77.3 years.
This good news, however, presents a challenge: keeping many more older people healthy than ever before. By 2050, the number of people aged 65 and above is expected to reach 1.6 billion, up from 761 million in 2021, according to the U.N.’s World Social Report 2023.
Alzheimer’s disease is one of the gravest threats to this growing population: as more and more people live longer and longer, the total number of people with Alzheimer’s disease worldwide is expected to increase by more than 150 percent in the next 30 years. These people have progressively greater challenges in carrying out their day-to-day activities, are more likely to become injured from falls, and face major challenges managing otherwise straightforward medical problems. Many people with Alzheimer’s disease suffer from hallucinations, confusion, and depression. It is also an ultimately fatal disease.
Alzheimer’s disease can cause horrible suffering among patients and their caregivers. This suffering is part of the large burden Alzheimer’s imposes on people and their families, public-health systems, and nations. The economic cost of this burden is difficult to assess. It involves not only easily quantifiable effects such as treatment and long-term care costs and loss of work productivity and lifespan, but also myriad others that are not easy to measure, such as its effects on the mental health and livelihoods of caregivers and other indirect medical costs.
Quantifying the broad economic cost of Alzheimer’s disease is important, not least because it is needed to assess the soundness of the expense to bring tests and, eventually, treatments to so many people through health systems. To this end, we have undertaken a comprehensive analysis, drawing on data from the Institute for Health Metrics and Evaluation (IHME), a leading research organization specializing in analyzing the global burden of diseases, as well as from other organizations and prior studies. We used a methodological approach that estimates the economic burden of Alzheimer’s disease based on people’s willingness to pay to avoid the risk of death. We also developed a macroeconomic model of the productive capacity of a country’s economy that allows for a reduction in labor and capital formation resulting from the disease burden. These methods take into account a wide array of direct and indirect costs of Alzheimer’s disease for individual patients, caregivers, and the aggregate economy.
Based on our willingness-to-pay approach, we estimate that the global economic burden of the disease in 2019 was roughly $2 trillion. By 2050, that burden will rise sharply to about $10 trillion and perhaps as high as $13.5 trillion. For comparison, world GDP is projected to be $228 trillion (inflation adjusted) in 2050.
The problem is especially urgent because the economic burden of Alzheimer’s is projected to worsen global economic inequality. Although the current economic costs of Alzheimer’s are concentrated in the wealthiest countries, these will grow much faster in low- and middle-income countries, which are less able to shoulder them. Between now and 2050, the number of people with Alzheimer’s disease in Northern Africa, the Middle East, and Eastern Sub-Saharan Africa is projected to grow by 250 percent, compared to 150 percent worldwide.
The economic burden of Alzheimer’s disease will be staggering and justifies a substantial scale-up of investments: in R&D spending on Alzheimer’s prevention, early detection, and disease-modifying therapies; the development of new modalities of care; and widespread, equitable access to these innovations. Experts in public health, medicine and policy are beginning to sound the alarm. They warn that massive investments are needed—not just for humanitarian reasons but also as wise economic policy.
The current toll
Disease burden research and estimates have typically focused on dementia, which, in addition to Alzheimer’s disease, also includes vascular dementia, frontotemporal dementia, Lewy body dementia, among other forms. Dementia is the sixth-leading source of disability burden among those aged 55 and older. Alzheimer’s disease accounts for approximately 60 to 80 percent of this burden. Based on this proportion, estimates from IHME suggest that in 2019, 34 million to 46 million people around the world had Alzheimer’s. Even if the age- and sex-specific prevalence of dementia remains stable in the coming years, population growth and rising longevity mean more people will be at risk of Alzheimer’s disease. In this vein, the total number of people living with Alzheimer’s disease is projected to rise to about 107 million by 2050. (For this estimate and some others in this article, we give the midpoints of projected ranges; in this case, the range was between 91 million and 122 million.)
Despite decades of research, medical interventions that substantially prevent, slow the progression of, or cure Alzheimer’s disease are not yet available. While there are many successful models of coordinated care for people with Alzheimer’s disease, they are not widely implemented. Most face difficult decisions about trying to receive care at home or living in a nursing home, both marked by hospitalizations and visits to the doctor.
A traditional method for tabulating these expenses is the cost-of-illness (COI) approach, which includes out-of-pocket medical and long-term care expenditures for patients and costs incurred by such payers as insurance companies or government-run healthcare systems. Globally, an estimated $184 billion was spent in direct healthcare costs for people with Alzheimer’s disease in 2019. By 2050, that number is projected to reach $1.1 trillion per year. While high-income countries are expected to see direct costs from Alzheimer’s disease multiply by a factor of five during this time, upper-middle income, lower-middle income, and low-income countries could see these COI estimates grow by factors of 21, 15, and 13, respectively.
A 2018 study using the cost-of-illness approach estimated the combined direct and indirect global costs of Alzheimer’s, including costs associated with seeking medical care (such as transportation and meals), the value of lost economic productivity of patients and caregivers, the medical cost of treating dementia-induced events (such as injuries) and, finally, the cost of the mental suffering of caregivers. The authors estimated the combined direct and indirect global costs of Alzheimer’s disease to be between $575 billion and $766 billion in 2015 and projected that it would increase to about $6.4 trillion by 2050.
COI studies, however, have limitations. Most leave out what economists call “productive nonmarket activities,” which include caring for grandchildren, volunteering in the community, and other unpaid activities that enrich lives. These activities are not measured traditionally in terms of wages or economic output and are rarely included in conventional estimates. In the U.S., 46 percent of a person’s total lifetime economic output is estimated to come after the age of 60—about half of it from employment, half from unpaid activities. Because of lack of data, analyses of the cost of care for Alzheimer’s disease, even if they include formal productivity losses for patients and caregivers, typically do not capture these nonmarket activities.
The economic costs of Alzheimer’s disease go even further beyond the cost of treatment or long-term care, as we and our colleagues reported in npj Aging in February 2024. A patient may no longer be physically or mentally capable of being employed or be less productive at work, both of which can reduce their own earnings and, in turn, the aggregate economic output of a country. Moreover, in many communities, especially among low-income families that cannot afford expensive nursing homes or home visits by professional caregivers, unpaid caregiving by family members (especially women) remains the norm, likely exacerbating income inequality. In fact, as many as 94 percent of people with dementia in low- and middle-income countries such as Brazil, China and Costa Rica receive their care at home. To quantify the lost economic productivity of unpaid caregivers, some researchers have calculated opportunity cost (potential earnings of the caregiver if they were instead gainfully employed in the market), and others have calculated replacement cost (the economic value of an equivalent amount of care that could be given by a professional caregiver at home instead of the unpaid caregiver). For a person with dementia in the U.S., these indirect cost estimates range from 31 to 49 percent of the total cost of care.
Even considering the lost market productivity of informal caregivers, other factors contribute substantially to the total cost of Alzheimer’s. Caring for people living with the disease is mentally and emotionally difficult for family members, but this pernicious impact is an indirect cost that is typically overlooked. Seeing your parent, grandparent or other loved one suffering from a debilitating disease like Alzheimer’s can be heartbreaking. The caregiver is often a witness to changes to their loved one’s personality and a deterioration of their memories—a decline that often leads to the inability to recognize family members. A comprehensive assessment of the economic burden of Alzheimer’s would include the toll on caregivers and other indirect costs.
A different way of looking at costs
Another method for measuring the potential economic burden of Alzheimer’s is called the value-per-statistical-life, or VSL. The VSL measures a society’s willingness to pay for lowering the risk of death. If a representative person were willing to pay $100 on average for a 1 in 100,000 reduction in the probability of one’s own death, it would take $10 million paid by 100,000 such individuals to collectively avoid one death (or to save one “statistical life”). VSL reflects how individuals value their own lives, presumably encompassing various aspects of healthy living that may range from having a job and earning money to being physically and mentally active, enjoying a good book, a vacation, or spending time with loved ones.
The VSL approach to assessing the economic burden of Alzheimer’s typically delivers estimates that encompass a broader range of value than estimates based on macroeconomic modeling or human capital and cost-of-illness approaches. But VSL-based estimates—like estimates based on these other methods—are subject to criticism.
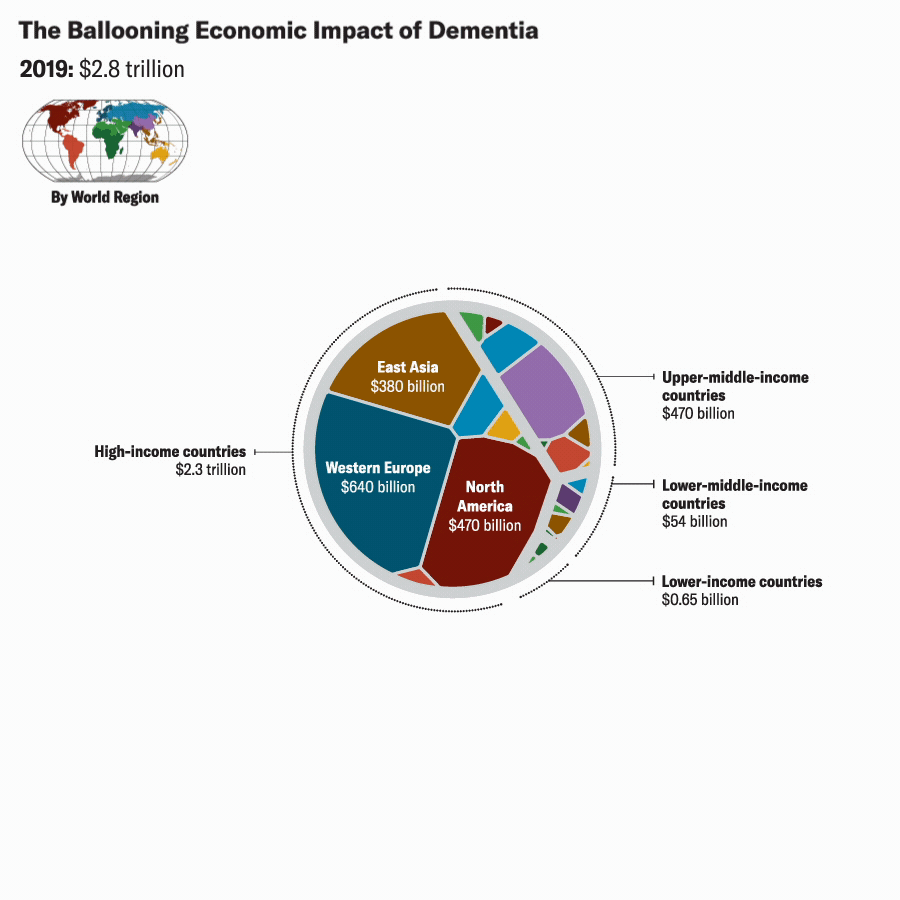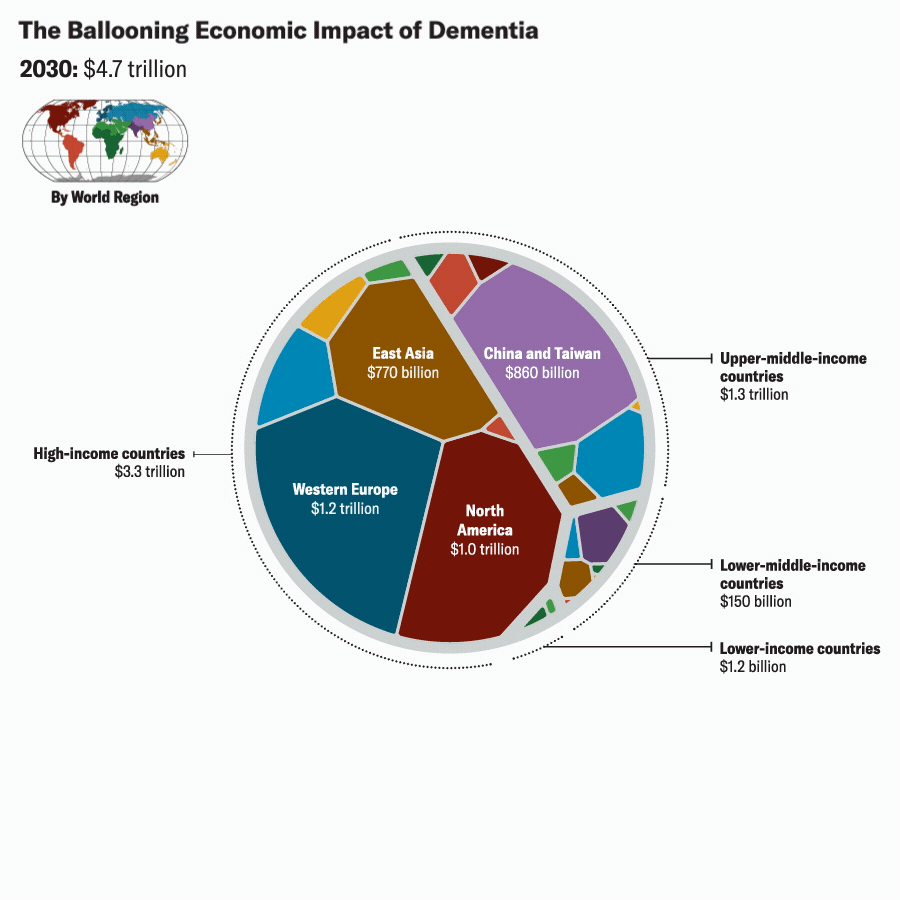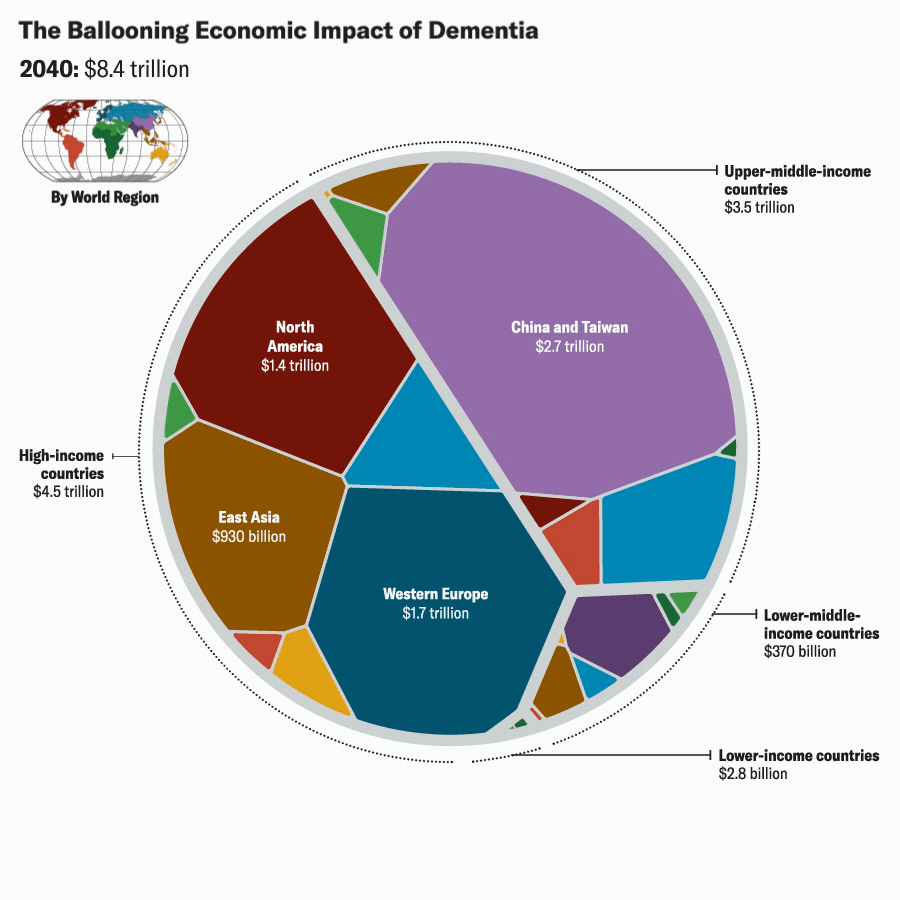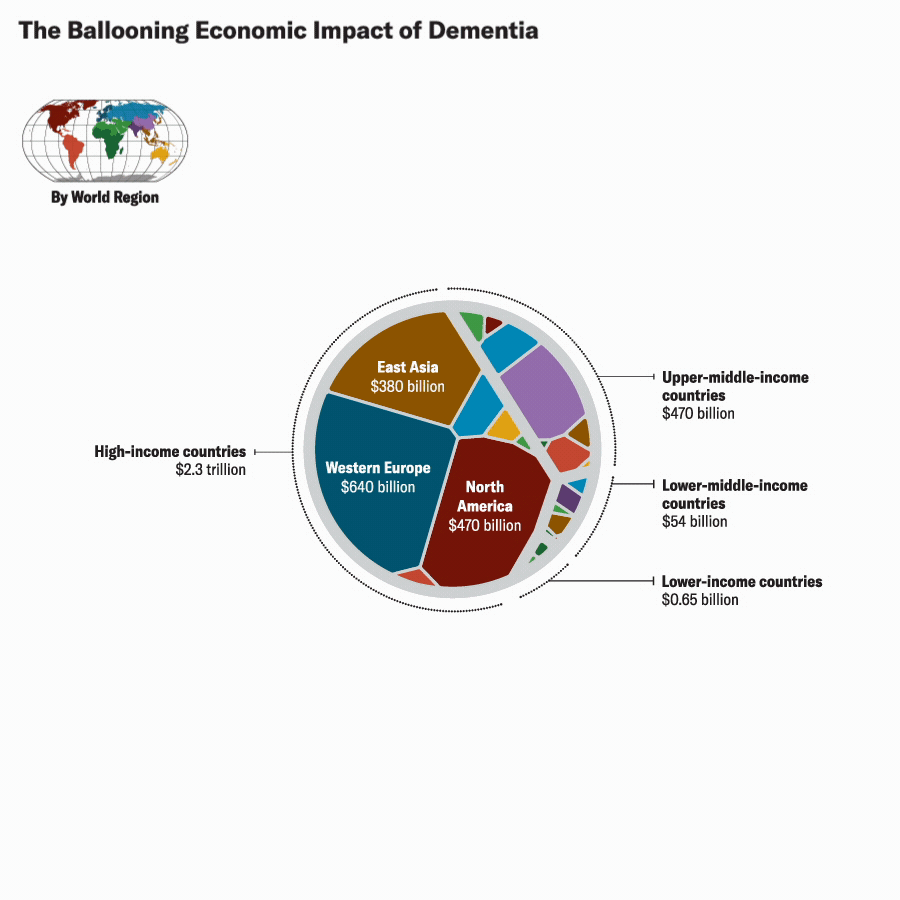
Katie Peek; Source: A. Nandi et al., eClinicalMedicine, September 2022, Vol.51:101580.
One common criticism is that VSL-derived cost estimates increase with income, suggesting that the lives of people living with Alzheimer’s disease in relatively high-income countries are worth more than the lives of people living with Alzheimer’s disease in low- or middle-income countries. This ethically problematic property of the VSL approach arises from the fact that VSL is derived from people’s willingness to pay for relatively small reductions in mortality, which in turn reflects their ability to pay.
This criticism can be addressed in multiple ways, such as by assuming that the value-per-statistical-life year (VSLY)—calculated as VSL divided by the expected number of years of life remaining—is the same for all countries. Modifications like this, however, are atypical in the literature on this topic. To keep our estimates broadly comparable to those of other economists, we chose not to make this adjustment to the VSL estimates in the study reported in this article.
Another criticism of the VSL approach is that it does not include all costs of Alzheimer’s disease, such as those associated with informal caregiving, medical research, or medical care paid for by third parties. This issue can, however, be addressed by interpreting VSL estimates as underestimates of the true costs or by separately calculating the excluded cost components and adding them to the VSL figures.
In a recently published study in eClinicalMedicine, we adopted the VSL approach to estimate the economic burden of Alzheimer’s disease and other forms of dementia across 168 countries containing more than 99 percent of the world’s population.
In this study, we obtained data on disability adjusted life years (DALYs) lost to Alzheimer’s disease from a database prepared by the IHME Global Burden of Diseases study. A DALY represents the loss of the equivalent of one full year of healthy life. DALYs are calculated as the sum of the years of life lost for those who die from Alzheimer’s and the effective years of life lost for those who experience disability from the disease. The more severe the dementia, the more effective years of life are lost. For example, one year of living with severe Alzheimer’s is equivalent to 0.6 year of living without the disease, and one year of living with mild disease is equal to 0.93 year of living without it.
We then monetized each DALY lost to Alzheimer’s disease as being equal to VSLY. For instance, to calculate the VSLY for the U.S., we started by considering the VSL for the U.S., which various U.S. government agencies and researchers have estimated to be approximately $10 million to $12 million. (For other countries, VSL is typically derived by adjusting the U.S. estimate for differences in income and purchasing power.) Based on standard life table estimates, a median-aged U.S. resident in 2019 would have a remaining life expectancy of 43 years. The VSLY figure for the U.S. comes to $246,512 ($10.6 million divided by 43 years). This figure represents the monetary value of each year of full health lost to Alzheimer’s in the U.S., which can be interpreted to include the value of all measurable aspects of a healthy year of life, such as employment, as well as intangible aspects, such as enjoying leisure. It likely does not capture the full economic value of unpaid caregiving, which constitutes a large part of overall care costs for Alzheimer’s.
We projected future VSLY values for each country based on average annual growth rates of gross national income per capita from 2010 to 2019. Based on these figures and our projections of Alzheimer’s cases (which assume that Alzheimer’s would continue to account for 60 to 80 percent of the future global burden of dementia, we estimate the global economic burden of Alzheimer’s disease in 2019 to be about $2 trillion. We also project that the global economic burden of Alzheimer’s would rise to between $2.8 trillion and $3.8 trillion in 2030, to between $5.1 trillion and $6.8 trillion in 2040, and to between $10.1 trillion and $13.5 trillion by 2050. These estimates are reported in 2020 U.S. dollars, with future values discounted at the rate of 3 percent per year.
Our findings also show that the global center of gravity of the economic burden of Alzheimer’s will gradually shift away from high-income countries, which currently have the greatest number of people with Alzheimer’s and toward upper-middle-income countries, which will experience rapid growth in the number of older adults and, as a result, those living with Alzheimer’s. Between 2019 and 2050, the VSL-based economic burden is projected to increase by a factor of 22 in upper-middle-income countries, as compared to 3 in high-income countries. The burden will also grow in other parts of the world at greater rates than in high-income countries, though their absolute magnitude in 2050 will remain low in comparison with upper-middle-income countries.
While the two approaches for assessing the economic burden that we’ve discussed thus far—the COI method and VSL method—can reliably estimate the economic impact of Alzheimer’s disease on people with the disease and their caregivers, neither account for its longer-term and aggregate macroeconomic effects. Deaths and disability from Alzheimer’s disease diminish the size and productivity of the workforce, reducing national economic output.
Out-of-pocket care costs from Alzheimer’s disease can significantly deplete household savings. These savings might otherwise have been invested in retirement accounts or used to support family businesses or the education of children or grandchildren. Costs incurred by health insurance companies translate to higher insurance premiums and lower household savings for consumers. Similarly, costs paid by social healthcare systems may need to be funded by taxes that, in turn, reduce personal savings. Finally, diverting money to Alzheimer’s care might reduce vital public investments in education, other aspects of health, and infrastructure development that generally have high economic returns.
Our macroeconomic model
To account for the effects of Alzheimer’s disease on national and global economies, we developed a macroeconomic model that simulates the productive capacity of a country’s overall economy. It accounts for the reductions in labor from Alzheimer’s-related mortality and morbidity, both for patients and caregivers, and reductions in capital formation because of lower savings. It iteratively estimates the value of economic output based on the available labor and capital stock, part of which is then saved by households and invested as capital to spur future economic production.
We estimated that during the 2020-2030 decade, loss of labor and lower rates of capital formation would lead to a global macroeconomic cost of $1.5 trillion. We project cumulative losses between 2020 and 2040 to be about $4.3 trillion and losses from 2020 to 2050 to be about $7.3 trillion. These estimates are conservative, however, in that they do not account for likely economy-wide technological progress, and they do not include the value of productive nonmarket activities of people with Alzheimer’s disease.
Macroeconomic analyses provide a fundamentally different view from COI or VSL on the potential future economic burden of Alzheimer’s disease. All take into account demographic and disease-burden patterns, but macroeconomic analyses also account for the underlying rates of household savings, capital formation, and average income in each country. Our macroeconomic analyses find that while low- and middle-income countries are projected to bear the largest health burdens—close to three-quarters of the DALYs lost to Alzheimer’s disease by 2050—their share of the macroeconomic burden would remain just under 50 percent. As another example, South Asia would still account for 19.4 percent of the DALYs lost to Alzheimer’s in 2050 but only 1.6 percent of the macroeconomic burden from 2020 to 2050, while North America would account for only 4.5 percent of the DALYs in 2050 but 28.5 percent of the economic loss from 2020 to 2050.
Like our willingness-to-pay (VSL) analysis, our macroeconomic burden modeling uses underlying disease-burden projections that differ from the latest IHME estimates. Our projections assume that the Alzheimer’s disease burden would continue to grow in each country at the same rate as in the past (from 2010 to 2019). By contrast, IHME’s estimates are based on a more detailed methodology that considers several contributing or risk factors for dementia, such as education level, physical activity, and exposure to air pollution. However, the IHME estimates are available only for 2050 and not the intermediate years that we evaluated. The IHME study also does not directly report the DALYs that are used in our analyses. Our linear projections of future Alzheimer’s cases are slightly lower than IHME estimates: 133 percent between 2019 and 2050 versus 166 percent in IHME estimates. This suggests that our estimates of the economic burden of Alzheimer’s disease may be relatively conservative. While our estimates may be limited by some uncertainty surrounding the future disease burden, they nonetheless present a strong economic case for investing in research to prevent and manage Alzheimer’s disease.
To be sure, recent research has shown that wealth and health are a two-way street. Population health is a benefit of affluence, and healthy populations tend to have more vibrant and robust economies than their less healthy counterparts. Healthy populations also tend to be more stable, cohesive, equitable, and secure. Insofar as global societal well-being is driven by population health in a plethora of ways, the full benefits of population health need to be acknowledged and measured in the interest of efficiently allocating scarce public and private resources and garnering the biggest returns from them.
The combination of a rapidly aging global population and the lack of treatments for Alzheimer’s is problematic now. In the coming decades, as the toll of the disease swells rapidly, it could become a significant impediment to human progress. In 2020, the U.N. General Assembly declared this decade as the Decade of Healthy Aging, calling upon governments, civil society organizations, the private sector, academic experts, and other stakeholders to join hands in improving the lives of older adults. It would be wise to act now.
This article is part of The New Age of Alzheimer’s, a special report on the advances fueling hope for ending this devastating disease.