As more data on COVID-19 vaccines became available, we started conducting an ongoing systematic review of literature related to vaccine efficacy and estimated the effectiveness of all vaccines against infection, symptomatic and severe disease (hospitalization and death), and how that effectiveness wanes over time, regularly updating the estimates as new data and variants emerged.
“Vaccine efficacy” is not a single number – we capture:
- Prevention of infection: a vaccine’s efficacy at stopping transmission of the virus from one person to another. An exposed person will not contract the virus, and by definition they will also not develop symptoms or disease.
- Prevention of severe disease: a vaccine’s efficacy at preventing an exposed person from developing serious symptoms that often require hospitalization and lead to death.
For each of the outcomes above, we also capture the differences by:
- Baseline vaccine effectiveness after second dose, waning immunity considering time since vaccination, and booster dose efficacy;
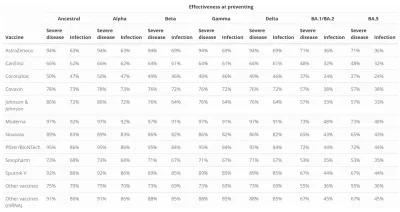
- Vaccine type: Pfizer & BioNTech (BNT162b2), Moderna (mRNA-1279), AstraZeneca (ChAdOx1), Janssen (Ad26.COV2.S), and other vaccines with available results;
- Variant of COVID-19: D614G (ancestral type), B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and B.1.1.529 (Omicron).
We have reviewed peer-reviewed publications, reports, preprints, medRxiv, and news articles since June 2021, and these are continually being searched, in any language.
We have performed data synthesis through a descriptive synthesis, with a summary of each included study. The quantitative synthesis has been performed through a meta-regression—Bayesian, regularized, trimmed (MR-BRT) using splines. We have updated estimates of vaccine efficacy against infection and severe disease from the Delta variant using 10 studies covering six countries. To estimate waning protection from vaccination, we systematically compiled 20 studies from nine countries that estimated vaccine efficacy as a function of time since the second dose. In addition, to date we have found 10 studies covering six countries for the Omicron variant and eight studies covering six countries for booster doses.
The most recently updated table of vaccine efficacy is included in our results briefing documents and in the table that follows. Results for waning immunity can be found on the page entitled COVID-19 model update: Omicron and waning immunity.
COVID-19 Projections